1 Tnf-ãžâ± and Neuropathic Pain - a Review
Abstract
Patients with chronic pain normally suffer from working retentivity deficits, which may decrease their intellectual ability significantly. Despite intensive clinical studies, the mechanism underlying this course of retention impairment remains elusive. In this study, we investigated this consequence in the spared nerve injury (SNI) model of neuropathic pain, a most common course of chronic pain. We establish that SNI impaired working memory and short-term memory in rats and mice. To explore the potential mechanisms, nosotros studied synaptic manual/plasticity in hippocampus, a brain region critically involved in memory function. We plant that frequency facilitation, a presynaptic class of short-term plasticity, and long-term potentiation at CA3–CA1 synapses were impaired after SNI. Structurally, density of presynaptic boutons in hippocampal CA1 synapses was reduced significantly. At the molecular level, we found that tumor necrosis factor-α (TNF-α) increased in cerebrospinal fluid, in hippocampal tissue and in plasma afterwards SNI. Intracerebroventricular or intrahippocampal injection of recombinant rat TNF mimicked the effects of SNI in naive rats, whereas inhibition of TNF-α or genetic deletion of TNF receptor one prevented both memory deficits and synaptic dysfunction induced by SNI. As TNF-α is critical for evolution of neuropathic pain, nosotros suggested that the over-production of TNF-α following peripheral nerve injury might atomic number 82 to neuropathic hurting and retention deficits, simultaneously.
INTRODUCTION
Compelling clinical evidence has shown that chronic pain patients (without traumatic brain injury or neurological disorder) commonly endure from memory deficits (Hart et al, 2000; Legrain et al, 2009). Around 2-3rd of patients with chronic pain exhibit disruption of working memory (Dick and Rashiq, 2007). Psychologically, working memory is associated with power of people to remember and procedure short-term data for making decisions or solving bug. It has been shown that intact working memory is critical for achievements of an individual in modern life (Alloway et al, 2009).
Although numerous clinical studies have been carried out to elucidate why chronic pain patients have memory deficits, the results are controversial. Some studies advise that retention impairment may be associated with intensity of pain (see Hart et al, 2000 for a review). However, other studies show that acute hurting in health individual is non always associated with cerebral deficit (Etherton et al, 2006) and that the relief of pain in chronic pain patients with opioids fails to improve their cognitive office (Dick and Rashiq, 2007). Thus, the mechanisms underlying the memory deficits in chronic pain patients remain elusive.
To explain why chronic pain patients have retentiveness deficits, two attractive only different theories take been raised. Eccleston (1995) has suggested that attention, which is closely relevant to working memory (Awh et al, 2006), is a finite and unitary resource and hurting-related sensory inputs compete for the limited attention resources, thereby affecting the working retentiveness process that involves the processing and integrating of other information. Hence, disruption of attention by pain may lead to retentivity deficits. Alternatively, Hart et al (2000) propose that the chemic substances produced in chronic pain weather may influence function of neural circuitries, leading to the impairment of attention and retention. Therefore, memory impairment may be not resulted from hurting, but rather from unknown sources.
The well-nigh common course of chronic pain is neuropathic hurting (Toth et al, 2009), which is initiated or caused past a primary lesion or dysfunction in the nervous system (International Clan for the Study of Hurting, 1986). Information technology has been repeatedly demonstrated that the upregulation of proinflammatory cytokines following nerve injury accept a key function in the development of neuropathic pain (George et al, 2004; Noguchi et al, 2004; Shamash et al, 2002; Yamanaka et al, 2004). Tumor necrosis factor-α (TNF-α), a key pro-inflammatory cytokine, increased substantially in dorsal root ganglia (DRG), spinal dorsal horn, and hippocampus following peripheral nerve injury (Ignatowski et al, 1999; Spengler et al, 2007; Xu et al, 2006) and inhibition of TNF-α synthesis (George et al, 2000; Ribeiro et al, 2000; Xu et al, 2006) or antagonism of TNF receptor one (TNFR1; Schafers et al, 2001; Sommer et al, 2001) attenuates the behavioral signs of neuropathic pain.
In this study, we explored the retention function in the spared nerve injury (SNI) model of neuropathic pain (Decosterd and Woolf, 2000) and found that indeed, working memory or curt-term retention was impaired severally afterward SNI. As synaptic density and plasticity in hippocampus are disquisitional for memory formation (Bruel-Jungerman et al, 2007; Gould and Leuner, 2010; Leuner and Shors, 2004), we studied the synaptic transmission and plasticity in hippocampus in brute with SNI and found that both short-term and long-term synaptic plasticity was impaired. Finally, nosotros showed that the increase in TNF-α, which induces neuropathic pain, led to retention deficits following peripheral nerve injury.
MATERIALS AND METHODS
Animals
Adult male person Sprague–Dawley rats (220–260 g), adult male C57 mice (22–27 g) from Institute of Experimental Animal of Lord's day Yat-sen University and adult male TNFR1-knockout (KO) mice weighing 22–27 g from the Jackson'south laboratory were used. The animals were housed in separated cages with access to food and water advertisement libitum. The room was kept at 23±1°C and 50–60% humidity, nether a light cycle (0600 to 1800 hours). All experimental procedures were approved by the Local Fauna Care Committee.
Spared Nerve Injury
The SNI procedure was carried out following the procedures described past Decosterd and Woolf (2000). Nether anesthesia with chloral hydrate (0.4 grand/kg, i.p.), an incision through skin on the lateral surface of the thigh was made, and the biceps femoris muscle was dissected bluntly to expose the left sciatic nerve and its three terminal branches. The common peroneal and the tibial fretfulness were tightly ligated with five-0 silk and transected distal to the ligation, removing a 2–iv mm length of each nervus. Great care was taken to avoid whatever contact with or stretching of the intact sural nervus. The wound was closed in two layers.
Measurement of Mechanical Allodynia
Mechanical sensitivity was assessed using von Frey hairs with the up-down method every bit described previously (Chaplan et al, 1994). Briefly, the animals were placed under separate transparent Plexiglas chambers positioned on a wire mesh flooring. Five minutes were allowed for habituation. Each stimulus consisted of a 2–three s application of the von Frey hair to the lateral surface of the foot with a 5 min interval between stimuli. Quick withdrawal or licking of the manus in response to the stimulus was considered a positive response. The paw withdrawal threshold was monitored before and afterwards SNI, and before electrophysiological recordings or before the animal was killed for immunohistochemistry or TNF-α bioassay.
Eight-Arm Radial Maze Exam
To evaluate the working memory and reference memory in animals with SNI and sham performance (10–20 day group and 30–twoscore mean solar day group), the viii-arm radial maze exam was performed following the method described by Zou et al (1998). The animals were singly housed and kept on a restricted diet and body weight was maintained at 85% of their complimentary-feeding weight, with h2o beingness available ad libitum The radial maze consisted of a central octagonal platform (26 cm in diameter), from which 8 arms (67 cm long, twenty cm deep, x cm wide) radiated. At the end of each arm in that location was a 3 mm deep nutrient loving cup (iii mm in bore) that was invisible from the center of the maze. The experimental room was brightly lit and busy with conspicuous extra-maze cues. The cues remained constant throughout the experiment.
The animals were first habituated to eat food pellets placed in all arms of the radial maze. Once all the animals were running freely through the maze and readily consuming the pellet rewards at the first entry of each arm, memory testing began. During the tests, only four of viii artillery were baited with one 100 mg (for rat) or 15 mg (for mouse) in subconscious food pellet. The four baited artillery were allocated such that two of these arms were adjacent, and the other artillery were 90° apart from these arms (eg, arms 1, 3, six, and 8) and that were kept constant throughout the x days of testing for each fauna. The trial commenced with placement of the animal on the fundamental platform and was deemed finished equally soon as the food pellets had been consumed or when ten min had elapsed, whichever occurred beginning. At the end of each trial, the maze was cleaned. The number of arm entries was recorded until the trial was finished. The maze was rotated periodically to prevent the animals from using intra-maze cues to solve the task. The locomotion of each animal was monitored during the whole retentivity test and the information is shown in Supplementary Figure 1.
Entry into an unbaited arm was scored as a reference memory error. This reflects retention of information, which remains constant across trials and is a measure of long-term memory. Re-entry into a baited arm from which the nutrient pellet had already been consumed within a trial was scored as a working retentivity mistake. This mirrors the ability of the animal to retain short-term data for a unmarried trial, ie, to temporarily hold information online, and is a measure of working retentiveness. The animals, which entered less than 3 artillery or more than twenty arms in the outset ii min of the habituation phase, were considered inactive or excessively active and were excluded from the test. The tests were performed in 2 different cohorts of rats: 11–20 days and 31–twoscore days after SNI or sham functioning.
Electrophysiological Recording of Field Excitatory Postsynaptic Potentials in CA3–CA1 Synapses In Vivo
Following the procedures described previously (Xu et al, 1997), animals anaesthetized with urethane (i.5 one thousand/kg, i.p.) were placed in a stereotaxic frame. Field excitatory postsynaptic potentials (fEPSPs) were recorded from the stratum radiatum in CA1 following electrical stimulation of the Schaffer collateral–commissural pathway. Electrophysiological criteria (Leung, 1979) were used to determine the optimal electrode placement. The recording electrode was positioned 3.4 mm in rat (2.3 mm in mouse) posterior to bregma, 2.5 mm in rat (ane.75 mm in mouse) lateral to midline, and the depth of recording electrode was about 2.2 mm in rat (1.6 mm in mouse) from dura. The stimulating electrode was positioned 4.2 mm in rat (1.vii mm in mouse) posterior to bregma and 3.viii mm (ane.6 mm in mouse) lateral to midline, and nigh four.7 mm in depth in rat (1.8 mm in mouse) from dura. The positions of recoding electrode and stimulation electrode in a representative experiment in rat are shown in Supplementary Figure 2c–d. A single square pulse of voltage at low frequency (0.066 Hz, 0.2 ms duration) was used to evoke fEPSPs and the intensity of the test stimulus was adjusted to produce l–55% of maximum response. High-frequency stimulation (HFS, 100 Hz, l pulses, four trains at 15 south interval) protocol was used to induce long-term potentiation (LTP). The intensity of HFS was raised to evoke 75% of maximum fEPSPs amplitude. The amplitudes of fEPSPs were determined on-line past LTP program (http://www.ltp-program.com). In each experiment, the responses to twenty sequent test stimuli were averaged. The hateful amplitudes of responses earlier HFS served as baseline. The recordings were made before and in different time periods after SNI or sham performance (Effigy 2).
To measure out the frequency facilitation in CA1 synapses, a presynaptic form of short-term plasticity (Sun et al, 2005), 100 conditioning stimuli at 2, 4, and viii Hz were in turn delivered in the aforementioned animal at 20 min intervals. Stable baseline was recorded before conditioning stimuli. The intensity of conditioning stimuli was identical to test stimuli. The frequency facilitation was recorded at 20 days after SNI and sham operations.
The Quantification of Presynaptic Terminal Puncta and TNF-α
The detection of presynaptic terminal puncta with synaptophysin antibiotic in encephalon sections was performed as following. The image was collected with an Olympus Nine-70 confocal microscope using a × 60 h2o lens; water lens (Northward/A=1.2) at zoom × three, generating an paradigm with 78.half dozen × 78.six μm dimension. Serial z-sectioning was performed (thickness of 0.9 μm) and the best three z-sections (with highest number of puncta) were collected and merged into a unmarried image. The number of puncta in the CA1 region was estimated from the obtained images using Prototype-Pro-Plus software (Media Cybernetics). Groundwork levels were equalized and special filters to separate fluorescent puncta were practical. Settings, for each image, were adjusted to maximize the number of detected fluorescent puncta. Mean puncta number per 1000 μm2 was calculated. The difference in presynaptic terminal puncta density between each group should nevertheless be reliable, as all images were processed under the same conditions.
The sections stained with TNF-α antibody were examined with a Leica DFC350 FX (Leica Camera, Frg) fluorescence microscope and images were captured with a CCD spot camera. To quantify TNF-α immunofluorescence staining, the surface area of TNF-α-IR per section was measured in the hippocampus CA1 and CA3 areas using a computerized image analysis system (LAICA Qwin). A density threshold was set above background level to identify positively stained structures, and so the percentage of TNF-α-IR surface area in the whole tissue expanse was calculated. The brain sections were coded until the completion of data analysis.
Statistics
The information from the radial eight-arm maze, LTP, and frequency facilitation between groups were analyzed with repeated measures 2-way ANOVA, and mail service hoc test were used for detailed statistical analysis, as appropriate. For paw withdrawal threshold, non-parametric tests were used. The data betwixt testing days within group were analyzed with Friedman ANOVA for repeated measurements, followed by Wilcoxon'southward matched pairs test when appropriate, and the data betwixt groups on a given testing day were analyzed with the Mann–Whitney U-test. The results of immunohistochemistry and the novel object recognition test were analyzed with two-tailed unpaired Educatee'south t-tests. The data of TNF-α bioassay were analyzed using i-style ANOVA followed by individual post hoc comparisons (Tukey post hoc exam), whereas the information of TNF-α bioassay between ii groups was compared with two-tailed unpaired Student's t-tests. The correlation of two parameters in one group was analyzed with the Pearson'south test. All data are expressed as means±SEM. Statistical tests were carried out with SPSS 16.0 (SPSS, Somers, NY, USA). A P-value of <0.05 was considered significant.
RESULTS
Working Memory and Curt-Term Memory are Impaired in Rats Following SNI
To examination whether peripheral nerve injury, which often induces neuropathic hurting, may impair retentiveness function in adult rats, radial viii-arm maze tests were performed at 11–20 days after SNI and sham performance. The paw withdrawal thresholds were significantly decreased in the SNI group (Figure 1a, n=fifteen, F(6, 98)=53.95, P<0.01 compared with pre-operation, Friedman ANOVA), but not in the sham group (Effigy 1a and d, n=20, F(half dozen, 133)=two.44, P>0.05 vs preoperative baseline, Friedman ANOVA). The boilerplate working memory errors in the SNI rats with mechanical allodynia was significantly higher than those in sham group from the third to 10th test day (Figure 1b, F(1, 33)=24.46, P<0.05, n=15 in SNI grouping and n=20 in sham group, two-style ANOVA followed by mail hoc exam), whereas no difference in the average reference memory errors was detected between SNI group and sham group (Figure 1c, F(1, 33)=1.97, P>0.05, two-way ANOVA).
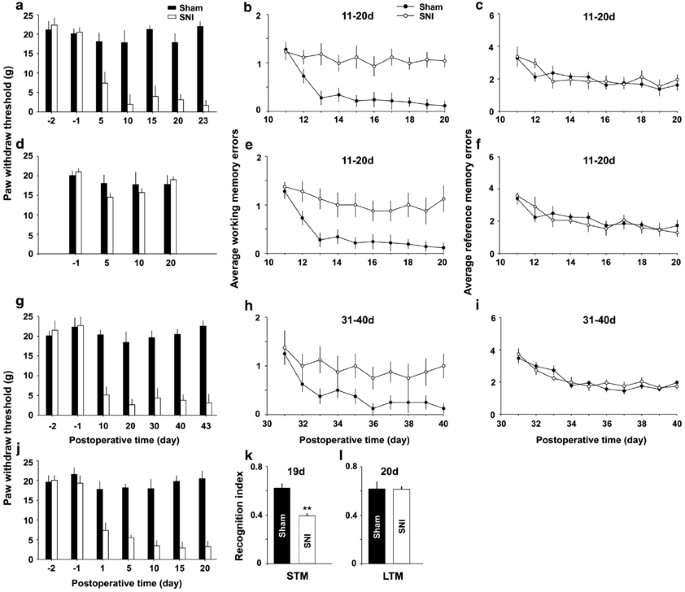
SNI impairs spatial working memory and brusque-term memory (STM), but not spatial reference retentivity or long-term retentivity (LTM) in rats. (a) The mechanical allodynia was verified by lasting decrease in paw withdrawal thresholds on ipsilateral side in SNI group, compared with those in sham group (north=fifteen in SNI group and n=20 in sham group). (b and c) In the same rats working memory and reference memory were evaluated with radial eight-arm maze at eleven–twenty days after operation. (d–f) In the v rats that did not exhibit significant decrease in paw withdrawal thresholds post-obit SNI, working memory errors but not reference memory errors were besides significantly higher, compared with sham group (n=20 in sham group). (yard–i) Paw withdrawal thresholds, working retention and reference retentivity in the rats at 31–forty days after SNI and sham performance are shown (n=10 in each group). (j–50) Hand withdrawal thresholds were tested in SNI and sham groups, and the recognition index for STM and for LTM were adamant by novel object recognition exam at xix days and at 20 days later on operation. ** P<0.01 compared with sham groups (n=8 in each group). Information are presented equally ways±SEM.
PowerPoint slide
To investigate the causal relation between mechanical allodynia and retentivity deficits, we tested memory function in another five rats that did not exhibit the subtract in paw withdrawal thresholds following SNI (Effigy 1d, n=5, F(six, 28)=0.64, P>0.05 compared with pre-operation, Friedman ANOVA). Surprisingly, working memory errors in these rats were also higher, compared with sham group (Effigy 1e, F(1, 23)=15.24, P<0.05, n=5 in SNI group, and northward=xx in sham group, two-way ANOVA), whereas the reference memory was not unlike from sham group (Figure 1f, P>0.05, F(ane, 23)=1.67, two-fashion ANOVA). The results suggest that mechanical allodynia might exist not direct relevant to the memory deficits produced by SNI.
In other 20 rats, the retentiveness office was evaluated at 31–40 days after operation. Paw withdrawal thresholds decreased in SNI group (Figure 1g, F(6, 63)=43.85, P<0.01 compared with pre-performance, n=10 in each group, Friedman ANOVA). We found again that the average working retentiveness errors were significantly higher in SNI group than in sham grouping from the 3rd test 24-hour interval (Figure 1h, F(1, 18)=12.04, P<0.05, northward=10 in each group, two-mode ANOVA followed by mail hoc exam), and there was no deviation in the average reference retentivity errors betwixt the two groups (Figure 1i, F(1, 18)=1.07, P>0.05, two-way ANOVA).
To investigate further the upshot of SNI on retention role, the short-term memory and long-term retention were individually evaluated using the novel object recognition test (NORT) on day 19 and twenty-four hours 20 later on operation (encounter Supplementary Methods). The recognition alphabetize for curt-term memory (10 min rotation) but not that for long-term memory (24 h rotation) in the same cohorts of rats was significantly lower in SNI grouping than in sham group (Figure 1k and 50, P<0.01, due north=8 in each group, 2-tailed unpaired Student's t-examination).
No pregnant difference in locomotion activity between SNI and sham groups was detected (Supplementary Effigy 1a–c and Supplementary Effigy 1j).
SNI Leads to a Delayed Inhibition of LTP in Bilateral Hippocampus
To explore the synaptic mechanisms underlying the memory impairment produced by peripheral nerve injury, we recorded LTP in hippocampus in vivo at different time points after SNI and sham-operation. We focused in the CA3–CA1 synapses, considering both CA1 and CA3 are important for working memory and novelty detection (Kesner, 2007; Vago and Kesner, 2008).
As information technology has been proposed that hurting may atomic number 82 to memory deficits (Eccleston, 1995), to exam whether the intensive nociceptive inputs produced by peripheral nervus injury may direct bear on synaptic transmission and plasticity in hippocampus, fEPSPs were recorded in vivo in either ipsilateral or contralateral hippocampus before and later SNI performed on left side. We constitute that SNI did non bear on the amplitudes of fEPSPs at ipsilateral CA1 (Figure 2a, F(2, 12)=0.918, P>0.05 vs preoperative baseline, n=5, one-way ANOVA) and the magnitude of LTP induced by HFS at ane h after SNI was not different from that in sham group (Effigy 2a, F(one, 8)=1.266, P>0.05, n=5 in each group, 2-fashion ANOVA). SNI also did not bear upon amplitudes of fEPSPs recorded in contralateral CA1 (Supplementary Effigy 2a, F(2, 12)=1.561, P>0.05 vs preoperative baseline, n=5, one-fashion ANOVA) and LTP induction (Supplementary Figure 2a, F(1, 8)=6.715, P>0.05, compared with sham group, n=5 in each group, two-way ANOVA). Whereas, in 18–twenty h afterwards SNI group, the potentiation past HFS returned to baseline within 1 h (Figure 2b, F(ane, 10)=29.81, P<0.05 compared with sham group, due north=6 in each group, two-mode ANOVA followed by post hoc test), indicating late-phase LTP was abolished. Furthermore, at 6–ten days subsequently SNI, the potentiation by HFS persisted for <30 min (Figure 2c, n=6, F(i, 10)=57.60, P>0.05 compared with sham grouping, two-fashion ANOVA followed past post hoc examination), suggesting LTP was completely blocked and only short-term potentiation remained.
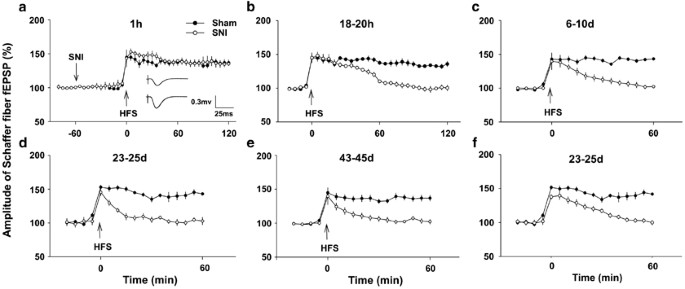
SNI inhibits LTP in CA3–CA1 synapses in a fourth dimension-dependent manner. (a) SNI did non affect the baseline of fEPSPs and LTP induction past HFS (100 Hz, 50 pulses, four trains with 15 south intervals), which was delivered at 1 h afterward nerve injury (northward=5 in each group). Insert: the raw traces of fEPSPs before (top trace) and afterwards (bottom trace) HFS. (b and c) The time courses of potentiation by HFS in 18–twenty h and 6–10 day SNI group (n=6 in each group). (d and e) The recordings were made post-obit behavioral test as illustrated in Figure 1a–c and Figure 1g–i (n=10–15 in each grouping). (f) The recordings were made in rats (due north=5 in SNI group and due north=xx in sham group) that did not exhibit allodynia but had working memory deficits, as shown in Figure 1d–f. Information are presented as ways±SEM.
PowerPoint slide
To determine the relationship between LTP inhibition and retentivity impairment, nosotros tried to record LTP in the rats that were used for radial maze retentiveness tests (shown in Effigy 1a–c and Figure 1g–i), and found that HFS failed to induce LTP in 23–25 twenty-four hours and 43–45 twenty-four hours SNI groups (Effigy 2nd and east, n=10–15 in each group). We also evaluated the synaptic plasticity in the rats that did non showroom mechanical allodynia but had working memory deficits (shown in Figure 1d–f), and plant that HFS besides failed to induce LTP in these animals (Figure 2f, n=v). Furthermore, HFS was unable to induce LTP in contralateral hippocampus at 20 days subsequently SNI (Supplementary Figure 2b), which was tested in other v rats. In dissimilarity, LTP persisted until to the end of experiments (up to 5 h) in all sham-operated groups (Figure 2, P<0.01 compared with baseline, i-way ANOVA, northward=five–20 in each time period). The delayed and bilateral inhibitory effect of SNI on hippocampal LTP suggested that peripheral nerve injury might impair memory function, indirectly.
SNI Reduces Both Presynaptic Boutons and the Frequency Facilitation at CA3–CA1 Synapse
As the amount of synaptic connections is critical for retentiveness function (Bruel-Jungerman et al, 2007), we compared the number of presynaptic boutons past calculating the density of synaptophysin-positive puncta (a vesicle protein) in stratum radiatum of CA1 betwixt SNI and sham rats, which had been used for novel object recognition examination (shown Figure 1j–l). The density of puncta was significantly lower in SNI group than that in sham group (Figure 3a and b, P<0.01, north=8 in each grouping, two-tailed unpaired Student's t-examination). Interestingly, we found that the density of puncta was positively correlated with recognition index for short-term memory in individual rats (Figure 3c, r 2=0.8746, P=0.0003, Pearson test). Thus, the reduction of presynaptic boutons might exist a structural footing of the memory impairment produced by SNI.
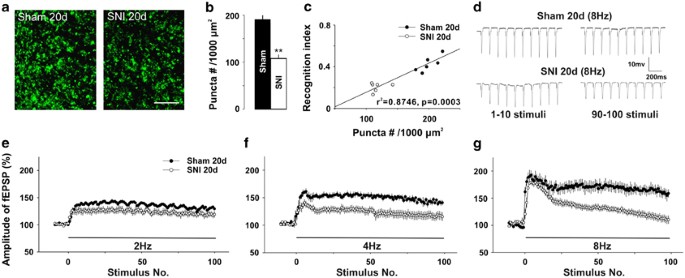
SNI reduces presynaptic final puncta density and frequency facilitation in CA1 region. (a) The representative images of presynaptic final synaptophysin puncta in CA1 from a 20-solar day-SNI rat and a sham-operated rat are shown. Scale bar=x μm. (b) The summary information of presynaptic concluding puncta densities in SNI group and sham grouping (n=eight in each group). (c) The correlation betwixt presynaptic terminal puncta density and the recognition index for short-term memory (r 2=0.8746, P=0.0003). (d) Original traces of fEPSPs evoked past eight Hz stimuli in a SNI rat and a sham-operated rat. (e–yard) The facilitation of fEPSPs produced by 2, 4, and 8 Hz stimuli in same SNI rats and in sham rats (n=5 in each group) in xx min intervals. The test stimulation was 0.066 Hz, and the intensity of conditioning stimuli was identical to that of test stimuli. ** P<0.01 compared with sham groups and information are presented as ways±SEM.
PowerPoint slide
As the reduction of the synaptophysin in presynaptic terminals may affect presynaptic release during synaptic transmission, we next tested whether the frequency facilitation, which is closely related to presynaptic neurotransmitter release (Sunday et al, 2005), might be inverse following SNI. The magnitude of facilitation induced past 2 Hz stimulation in SNI group was non dissimilar from that in sham group (Figure 3e, F(ane, viii)=0.21, P>0.05, due north=five in each grouping, two-way ANOVA). Notwithstanding, the facilitation induced by iv and 8 Hz were significantly lower in SNI rats than those in sham-operated rats (Figure 3f and thou, F(1, viii)=0.33, P<0.05 and F(one, 8)=three.48, P<0.01, respectively, n=5 in each grouping, ii-way ANOVA). Furthermore, in SNI group the facilitation induced past 2 and 4 Hz stimulation persisted during 100 stimuli (Figure 3e and f), whereas the facilitation induced by 8 Hz decreased progressively and returned to baseline at the terminate of stimulation (Figure 3d and g). The subtract in frequency facilitation in CA3–CA1 synapses following SNI might contribute to the impairment of working memory.
TNF-α is Increased in Cerebrospinal Fluid, Hippocampus, and Plasma Following SNI
Previous studies have shown that TNF-α is upregulated in DRG and spinal dorsal horn (Xu et al, 2006) post-obit nerve injury, and that TNF-α at pathological concentration inhibits LTP in hippocampus (Pickering et al, 2005) and impairs cerebral function (Tobinick, 2009). Appropriately, we tested whether TNF-α is increased in CSF, in hippocampal tissue, and in plasma at unlike time points following SNI (encounter Supplementary Methods). Indeed, the concentrations of TNF-α in CSF increased significantly at 5 h following SNI, reaching to superlative at 10 h, remaining at high level at vi days, 20 days afterward SNI (Figure 4a, F(five, 24)=64.02, P<0.01 compared with control (0 h), n=5 in each grouping, one-mode ANOVA followed by mail service hoc test). In hippocampal tissue, TNF-α did non modify immediately after SNI, but increased at 18 h after SNI and persisted for at least forty days (Figure 4b, F(five, 24)=16.10, P<0.01 compared with command (0 h), n=5 in each group, i-way ANOVA followed by mail hoc test). We found that the amplitudes of fEPSPs recorded at 30 min afterward HFS were negatively correlated with the concentrations of TNF-α in hippocampus (Figure 4c, r 2=0.9401, P=0.0001, Pearson examination) in SNI rats. The level of TNF-α in plasma also increased significantly at 3 days and at 7 days subsequently SNI, compared with control (Supplementary Figure three, F(2, 12)=21.53, P<0.01 compared with command (0 h), north=5 in each group, one-way ANOVA followed by post hoc test).
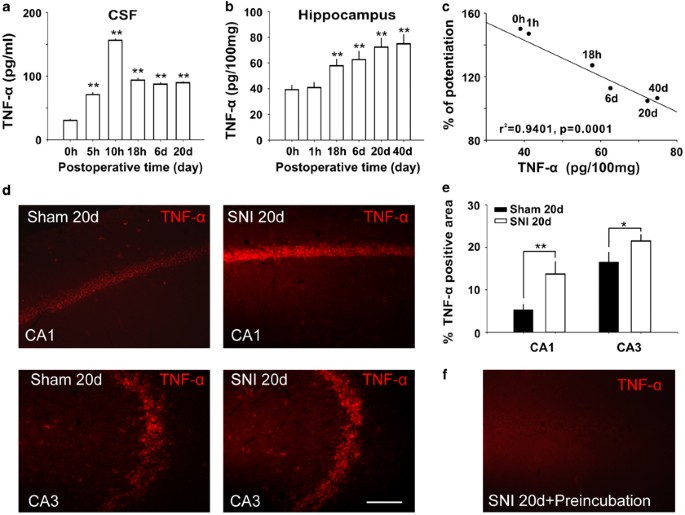
SNI increases TNF-α in cerebrospinal fluid and hippocampal tissue and the concentration of TNF-α in hippocampus is correlated with LTP inhibition. (a and b) The concentrations of TNF-α in cerebrospinal fluid (CSF) and in hippocampal tissue at different time points following SNI (n=5 at each time point). (c) The correlation analysis betwixt the amplitude of fEPSPs recorded at thirty min later on HFS and the concentrations of TNF-α in hippocampal tissue at different fourth dimension points following SNI (north=5–xx at each time indicate). ** P<0.01 compared with the time point of 0 h after SNI. (d) Representative experiments showed the difference in TNF-α-IR area in CA1 and CA3 betwixt sham operated and SNI rats. (eastward) The histogram showed the summary data (n=six) of TNF-α-IR-positive staining expanse in CA1 and CA3 in sham and SNI groups. (f) Identification of specificity of anti-TNF-α used in this study. The TNF-α-IR was clearly decreased in serial sections past preincubating anti-TNF-α antibiotic with TNF-α. Scale bar=50 μm. * P<0.05, ** P<0.01 vs sham groups. Data are presented every bit means±SEM.
PowerPoint slide
The expression of TNF-α in hippocampus was also accessed using immunohistochemistry method (see Supplementary Methods). Compared with sham group, the percent of TNF-α-IR-positive area in the hippocampal CA1 and CA3 was significantly higher in twenty day SNI group (Figure 4d and e, P<0.01 in CA1 and P<0.05 in CA3, n=6 in each groups, two-tailed unpaired Student'south t-exam), which is in line with a previous work that TNF-α mRNA is upregulated in hippocampal neurons post-obit peripheral nerve injury (Spengler et al, 2007). These information suggested that increased TNF-α following SNI might be the molecular events underlying the dysfunction of hippocampal synapses and the impairment of retentivity.
Intracerebroventricular or Intrahippocampal Injection of rrTNF Impairs Both Working Memory and Hippocampal LTP
To test the consequence of TNF-α on memory part directly, we investigated whether intracerebroventricular (i.c.v.) injection of rrTNF (i μg/ml, 5 μl, in 10 min, see Supplementary Methods) in naive rats is capable of impairing memory function and LTP in hippocampus. In control group the same volume of artificial CSF (aCSF) was injected. At iii days after cessation of injection, the memory function was evaluated with radial maze. Like to rats with SNI, the average working memory errors were significantly higher in rrTNF group than those in aCSF group (Effigy 5a, F(one, 14)=22.88, P<0.05, n=8 in each group, two-mode ANOVA), whereas there was no difference in average reference memory errors between the two groups (Figure 5b, F(1, 14)=v.49, P>0.05, 2-mode ANOVA). HFS was able to induce LTP at CA3–CA1 synapses in aCSF grouping but non in rrTNF group (Supplementary Effigy 4a). In addition, i.c.5. injection of rrTNF (1 μg/ml, 5 μl, in 10 min) in intact rats did not affect baseline of fEPSPs, and identical to the results observed in SNI animals, LTP was blocked at 60 min later injection (Supplementary Figure 4b). The results indicated that elevation of TNF-α in CSF via exogenous application could imitate the inhibitory effect of SNI on working memory and LTP in hippocampus.
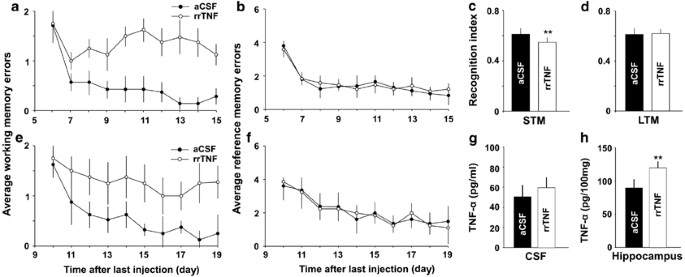
Intracerebroventricular or intrahippocampal injection of rrTNF mimics the effects of SNI. (a and b) Average working memory errors were college in rats with i.c.v. injection of rrTNF than in aCSF-treated rats, and there was no difference in reference memory performances between the 2 groups (n=8 in each group). (c–h) The experiments were performed in another cohorts of rats, in which rrTNF (200 ng/ml, 0.5 μl) or aCSF was infused into bilateral hippocampus for 3 successive days (n=viii in each grouping). (c and d) The recognition index for short-term memory only not that for long-term memory was lower in rrTNF-treated grouping than that in aCSF-treated group. (east and f) Average working memory errors were higher in rrTNF-treated group than that in aCSF-treated group, but at that place was no difference in reference retentiveness performances between two groups. (grand and h) The concentration of TNF-α in hippocampus simply not in CSF was higher in rrTNF-treated group, compared with that in aCSF-treated group (northward=4 in each group). ** P<0.01 vs aCSF-treated SNI group. Information are presented as ways±SEM.
PowerPoint slide
To test whether TNF-α may impair memory by acting in hippocampus but not in other brain regions, rrTNF (200 ng/ml, 0.5 μl, come across Supplementary Methods) or aCSF was injected into bilateral hippocampus for 3 successive days (daily), and 3 days after cessation of injection memory role was accessed with NORT and 8 arm maze tests. The index for short-term retention (10 min rotation) in rrTNF-treated group was significantly lower than that in aCSF-treated grouping (Figure 5c, P<0.01, n=8 in each grouping, two-tailed unpaired Student's t-exam). In that location was no difference in the index for long-term retentivity (24 h rotation) between the two groups (Figure 5d, P>0.05, two-tailed unpaired Student's t-exam). In radial eight-arm maze test, the average working retention errors in rrTNF-treated rats were significantly college, compared with those in aCSF-treated rats (Figure 5e, F(ane, xiv)=26.21, P<0.05, north=8 in each group, 2-way ANOVA), and no difference in reference memory functioning between the two groups was detected (Figure 5f, F(one, fourteen)=two.16, P>0.05, n=8 in each grouping, two-way ANOVA). There was no significant divergence in locomotion activity between rrTNF-treated group and aCSF-treated group (Supplementary Figure 1d–e and g). Post-obit the behavioral test, the levels of TNF-α in CSF and hippocampus were measured with ELISA. The concentration of TNF-α in hippocampus (Figure 5h, P<0.01, n=four in each grouping, ii-tailed unpaired Student's t-test) merely not in CSF (Figure 5g, P>0.05, northward=4 in each group, two-tailed unpaired Student'southward t-test) was significantly higher in rrTNF-treated group than in aCSF-treated group, suggesting that exogenous rrTNF may stimulate product of endogenous TNF-α in hippocampus.
Intracerebroventricular injection merely not intrahippocampal injection of rrTNF produced mechanical allodynia (Supplementary Figure 5).
Intraperitoneal Injection of TNF-α Synthesis Inhibitor or Intracerebroventricular Injection of TNF-α Antibody Prevents the Memory Harm Produced past SNI
Having demonstrated that TNF-α increased in CSF and hippocampus following SNI and exogenous awarding of rrTNF impaired synaptic plasticity and memory function, we side by side tested whether inhibition of the overproduction of TNF-α was sufficient to prevent the impairment of synaptic plasticity and memory induced past SNI. To do this, thalidomide (a TNF-α synthesis inhibitor), which has no effect on hippocampal LTP only prevents inhibition of LTP mediated by amyloid β peptide (Wang et al, 2005), was injected intraperitoneally (fifty mg/kg), starting at 2 h before SNI and so daily thereafter until day 7 after surgery. Like to our previous piece of work (Xu et al, 2006), injection of thalidomide prevented mechanical allodynia produced by SNI (Effigy 6a, P<0.05 compared with DMSO-treated group, north=eight–10 in each grouping, Isle of mann–Whitney U-exam). There was no difference in manus withdrawal thresholds between thalidomide-treated SNI rats and sham rats (Effigy 6a, P>0.05, Isle of mann–Whitney U-test). Importantly, nosotros institute that the average working memory errors in thalidomide-treated group were significantly lower than those in DMSO-treated grouping (Figure 6b, F(one, xiv)=24.46, P<0.05, two-way ANOVA), but were non dissimilar from those in sham group (Figure 6b, F(ane, 16)=1.97, P>0.05, 2-way ANOVA). Furthermore, LTP at CA3–CA1 synapses in the SNI rats treated with thalidomide was not different from that in sham-operated rats, whereas in SNI rats treated with DMSO HFS failed to induce LTP (Figure 6c). The results indicated that the damage of working memory and LTP past SNI was completely prevented by the pretreatment with thalidomide. To verify the increase in TNF-α was really prevented by thalidomide, CSF and hippocampal tissue were harvested following LTP recordings for assessment of TNF-α. The concentrations of the cytokine in both CSF and hippocampal tissue were significantly lower in thalidomide-treated SNI group than in vehicle-treated SNI group (Figure 6d and eastward, F(two, 23)=146.28 in CSF and F(two, 23)=20.71 in hippocampal tissue, P<0.01, one-way ANOVA followed by post hoc exam), and there was no deviation betwixt thalidomide-treated grouping and sham grouping (Figure 6d and e, P>0.05, one-style ANOVA followed by post hoc examination).
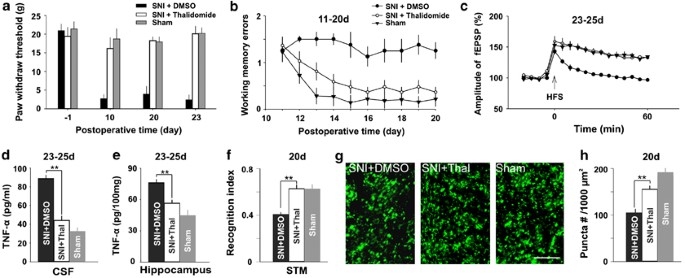
Inhibition of TNF-α synthesis by thalidomide prevents memory deficits and synaptic dysfunction produced by SNI. (a) Mitt withdrawal thresholds decreased in DMSO-treated SNI grouping but not in thalidomide-treated SNI group and sham group (n=8–10 in each group). (b) Average working memory errors in the thalidomide-treated SNI rats were lower than those in DMSO-treated SNI rats, and were not different from those in sham rats. (c) HFS induced LTP in thalidomide-treated SNI rats and sham rats simply not in DMSO-treated SNI rats. (d and e) The concentration of TNF-α in three groups of rats as indicated are shown. (f) The recognition alphabetize for short-term memory was significantly higher in thalidomide-treated SNI rats than that in DMSO-treated SNI rats. There is no difference between sham rats and thalidomide-treated SNI rats (n=8 in each group). (g and h) The density of presynaptic final puncta was higher in thalidomide-treated SNI group than that in DMSO-treated SNI group (due north=8 in each grouping). Scale bar=10 μm. ** P<0.01 compared with DMSO-treated SNI group. Information are presented as ways±SEM.
PowerPoint slide
In another prepare of experiments, we found that in SNI rats the recognition index for short-term retentivity detected by NORT was significantly lower in DMSO grouping than that in thalidomide group (Figure 6f, P<0.01, north=8 in each group, two-tailed unpaired student'due south t-examination), and the index in the SNI rats treated with thalidomide was not different from that in sham group (Figure 6f, P>0.05, two-tailed unpaired student'south t-exam). There was no significant departure in locomotion activity among three groups in the two behavioral tests (Supplementary Figure 1f and l). Furthermore, the density of presynaptic boutons in CA1 was also significantly lower in DMSO-treated SNI group than those in thalidomide-treated SNI grouping (Figure 6g and h, P<0.01, two-tailed unpaired pupil's t-test), and there was no divergence between sham rats and thalidomide-treated SNI rats (Figure 6g and h, P>0.05, two-tailed unpaired student's t-examination). The results suggested that the inhibition of overproduction of TNF-α might exist sufficient to prevent the structural and functional damage produced by SNI.
Every bit the inhibitory effect of thalidomide on TNF-α synthesis may be not specific, to confirm the effect of increased TNF-α on memory part, TNF-α antibiotic (250 μg/ml, 5 μl, run across Supplementary Methods) or aCSF was injected into cognitive ventricle at 2 h before and after SNI for 7 days (daily), and so memory function was evaluated with NORT and eight-arm maze tests. The recognition index for short-term memory (10 min rotation) was significantly higher in anti-TNF-α-treated group than that in aCSF-treated group (Figure 7a, P<0.01, n=7 in each group, two-tailed unpaired Student's t-test), but no difference in the index for long-term retention (24 h rotation) was detected between the two groups (Figure 7b, P>0.05, two-tailed unpaired Student'south t-test). Average working retentivity errors were significantly lower in anti-TNF-α-treated group, compared with aCSF-treated group (Effigy 7c, F(ane, 12)=22.38, P<0.05, n=seven in each grouping, two-fashion ANOVA) and there was no departure in average reference retention errors between the 2 groups (Effigy 7d, F(1, 12)=1.09, P>0.05, ii-way ANOVA). There was no significant difference in locomotion action between anti-TNF-α-treated SNI group and aCSF-treated SNI group (Supplementary Figure 1g and yard). The levels of TNF-α in both CSF and in hippocampus were significantly lower in anti-TNF-α-treated rats, compared with that in aCSF-treated rats (Effigy 7e and f, P<0.01, n=4 in each group, two-tailed unpaired Student's t-examination).
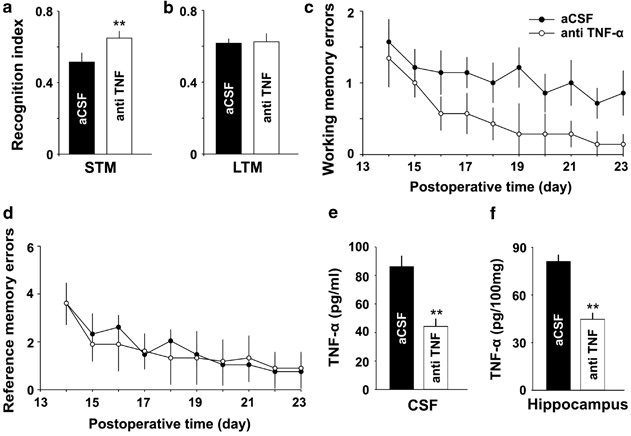
Intracerebroventricular injection of TNF-α antibody attenuates retentiveness damage induced by SNI. (a and b) The recognition index for short-term retentivity but not that for long-term retentivity tested at 10 days and at 11 days later on SNI was significantly higher in anti-TNF-α-treated group than that in aCSF-treated group (north=7 in each grouping). (c and d) Average working memory errors only non average reference memory errors accessed after SNI were significantly lower in anti-TNF-α-treated group, compared with aCSF-treated group (due north=7 in each group). (e and f) The concentrations of TNF-α in both CSF and in hippocampus were significantly lower in anti-TNF-α-treated rats, compared with aCSF-treated rats (n=iv in each group). ** P<0.01 vs aCSF-treated grouping. Information are presented as ways±SEM.
PowerPoint slide
The Effects of SNI on Retention Functioning and Hippocampal Synaptic Plasticity in Wide-Type Mice and TNFR1 KO Mice
To make up one's mind which subtype of TNF receptors is involved in memory deficits, nosotros performed the experiments with TNFR1 KO and wild-type (WT) mice at 20 days after SNI. In WT mice, we establish again that the boilerplate working memory errors in SNI grouping was significantly higher than those in sham grouping (Figure 8a, F(one, xiv)=20.32, P<0.05, northward=viii in each grouping, two-mode ANOVA). In contrast, in TNFR1 KO mice at that place was no difference in working memory errors between sham and SNI group (Figure 8f, F(1, 14)=34.24, P>0.05, north=8 in each group, two-way ANOVA). The potentiation past HFS persisted for at to the lowest degree two h (up to five h) in sham KO and sham WT mice, but for only 1 h in SNI KO mice and for xv min in SNI WT mice (Figure 8b and chiliad). The results indicated that in TNFR1 KO mice SNI did not affect working memory, but impaired belatedly-phase LTP.
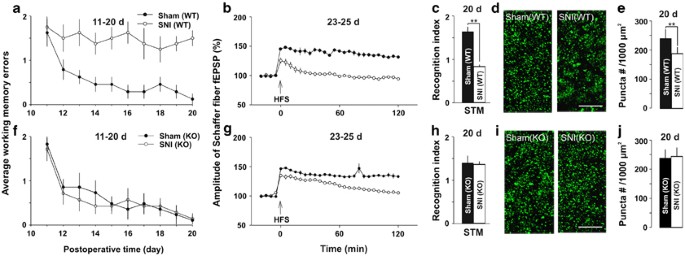
The differential effects of SNI on memory function and hippocampal synapses in wild-type (WT) and TNFR1-knockout mice. (a and b) In WT mice, working retentivity performance and LTP at CA3–CA1 synapses were impaired in SNI only not in sham-operation group (n=8 in each grouping). (c–eastward) The short-term memory index evaluated by NORT and presynaptic synaptophysin puncta density in CA1 are significant lower in SNI WT mice than those in Sham WT mice (n=8 in each group). (f) There was no difference between SNI KO mice and Sham KO mice in working memory performance. (g) The time course of potentiation induced by HFS in SNI KO mice and Sham KO mice, every bit indicated, at 23–25 days after operation (n=8 in each grouping). (h) Recognition alphabetize for short-term memory in SNI KO and sham KO mice are shown (northward=8 in each grouping). (i and j) The representative images show the presynaptic synaptophysin puncta in CA1 from KO mice after operation and the summary data of presynaptic final puncta densities in KO mice. Scale bar=x μm. ** P<0.01 compared with sham group and data are presented as ways±SEM.
PowerPoint slide
To further evaluate the part of TNFR1 in retention deficits in SNI mice, we investigated the effects of SNI on short-term memory measured with NORT, and the density of presynaptic boutons in WT and TNFR1 KO mice at 20 days later SNI and sham operation. The results showed that SNI dumb the short-term retention in WT mice simply non in TNFR1 KO mice, as in WT mice the recognition index for short-term retentivity was significantly lower in SNI grouping than that in sham grouping (Figure 8c, P<0.01, n=8 in each group, ii-tailed unpaired student's t-test), whereas in TNFR1 KO mice at that place was no difference between SNI group and sham group (Figure 8h, P>0.05, n=viii in each group, two-tailed unpaired educatee'due south t-test). In add-on, the cognition index was lower in sham-operated TNFR1 KO mice than that in sham-operated WT mice (Effigy 8c and h, ane.38±0.21 vs 1.59±0.fifteen, P<0.05, two-tailed unpaired student'south t-exam). The locomotion activeness in TNFR1 KO mice is not different from that in WT mice (Supplementary Figure 1h–i and n–o). Together, the deletion of TNFR1 might reduce curt-term memory slightly but prevent the memory deficits produced by SNI. In WT mice the density of presynaptic boutons was significantly lower in SNI mice, compared with that in sham-operated mice (Figure 8d and due east, P<0.01, 2-tailed unpaired educatee's t-test), whereas in TNFR1 KO mice in that location was no difference between SNI and sham groups (Figure 8i and j, P>0.05, two-tailed unpaired student's t-test). The results suggested that SNI reduced synaptic connections in WT mice, but non in TNFR1 KO mice. Consistent with the morphological finding, in TNFR1 KO mice the frequency facilitation induced by ii, 4, and 8 Hz in SNI group was not different from that in sham grouping (Figure 9a–d, P>0.05, n=5 in each group, two-way ANOVA). Thus, the increased TNF-α might exert the detrimental effect on memory role mainly past activation of TNFR1.
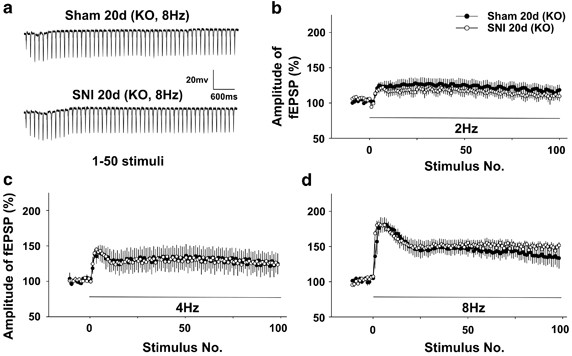
SNI does non affect the frequency facilitation in TNFR1 knockout mice. (a) The raw traces of fEPSPs evoked by 8 Hz stimuli (1–50 pulses) in SNI and sham-operated TNFR1 KO mice. (b–d) There was no deviation in frequency facilitation produced by 2, 4, and 8 Hz stimuli between SNI and sham groups in TNFR1-knockout mice (n=5 in each group). Data are presented every bit means±SEM.
PowerPoint slide
Word
In this work, we showed that SNI impaired the spatial working retention or short-term retention in rats and in mice. These data are consistent with the recent reports that spatial memory is reduced in SNI (Leite-Almeida et al, 2009) or spinal nerve ligation (Hu et al, 2010) models of neuropathic pain. These experimental data are in line with clinical studies that around ii-tertiary of chronic hurting patients have working memory impairment (Dick and Rashiq, 2007). Importantly, we demonstrated for the first time that the memory impairment produced by peripheral nerve injury was associated with dysfunction of the hippocampus, ie, the disruption of LTP and frequency facilitation at CA3–CA1 synapses, as well as the reduction of presynaptic boutons in hippocampal CA1 region. At the molecular level, peripheral nerve injury increased TNF-α in CSF, in hippocampus and in plasma. The increment in TNF-α was temporally correlated with the impairment of synaptic plasticity and memory. Intracerebroventricular or intrahippocampal injection of rrTNF mimicked the effects of SNI, whereas inhibition of TNF-α or genetic deletion of TNFR1 prevented both memory deficits and synaptic dysfunction produced by SNI. Our results suggested that peripheral nervus injury might lead to retentiveness deficits by impairing structure and function of hippocampus via upregulation of TNF-α.
Functional and Structural Damage of Hippocampus may Contribute to the Memory Deficits Following Peripheral Nerve Injury
Every bit mentioned in Introduction, it has been proposed (Eccleston, 1995) that hurting may pb to working memory deficits by competing express attention resource. The model is further extended recently (Legrain et al, 2009). However, the hypothesis is contradictory to the clinical findings that astute pain in health individuals does not produce cerebral deficit (Etherton et al, 2006), and that the relief of pain with opioids in chronic pain patients is incapable of improving cognitive function (Dick and Rashiq, 2007). In harmony with the clinical findings, the present piece of work showed that the impairment of memory and hippocampal LTP was evident not only in animals with mechanical allodynia but also in a modest part of animals that did not exhibit the behavioral sign of neuropathic pain following SNI. Moreover, SNI (cutting the common peroneal and the tibial fretfulness), which may activate the majority (if not all) of nociceptive afferents, had no acute effect on basal synaptic transmission and LTP consecration in hippocampus, suggesting that nociceptive inputs (hurting signals) may not impact synaptic manual and plasticity straight. Importantly, peripheral nerve injury induced a delayed LTP impairment in bilateral hippocampus. As curt-term potentiation (<thirty min) could still be recorded in the SNI rats, the failure of LTP induction may not be due to the saturation of synaptic potentiation, simply due to the impairment of synaptic plasticity. In improver, nosotros found that the presynaptic terminal puncta density was reduced and the frequency facilitation was inhibited following SNI. Altogether, our data suggested that the memory deficits produced by peripheral nervus injury might exist not resulted from nociceptive inputs only from dysfunction of synaptic connection in hippocampus.
The Increased TNF-α may Contribute to Both Neuropathic Pain and the Memory Deficits Following Peripheral Nervus Injury
In contrast to the theory past Eccleston (1995), Hart et al (2000) take proposed that the unknown molecules that disrupt neural circuitries underlying attention and memory may cause the cognitive deficits in chronic pain patients. Nosotros suggest that TNF-α may be one of these molecules, which may impair memory and induce neuropathic pain after peripheral nervus injury.
TNF-α tin be synthesized and released in the brain past glial cells and neurons, and exerts multifunctions by binding to 2 different TNF receptors (p55 or TNFR1 and p75 or TNFR2), which are constitutively expressed in nervous organization (Pickering et al, 2005). Previous studies accept revealed that TNF-α is upregulated in nervous system following peripheral nervus injury and the increased TNF-α is critical for the development of chronic neuropathic hurting (Ignatowski et al, 1999; Sekiguchi et al, 2009; Xu et al, 2006). Interestingly, in hippocampus, pathological concentration of TNF-α inhibits LTP (Butler et al, 2004; Cunningham et al, 1996; Pickering and O'Connor, 2007; Tancredi et al, 1992). In this report, we found that TNF-α increased in CSF, in hippocampus, and in plasma later on peripheral nerve injury, which was closely correlated with LTP inhibition. Both of the changes were detected at 18 h but not at one h after SNI. Intracerebroventricular or intrahippocampal injection of rrTNF mimicked the memory deficits and impairment in the part of CA1 synapses produced by peripheral nerve injury, whereas inhibition of TNF-α or genetic deletion of TNFR1 prevented the furnishings of SNI. These results suggest that the overproduction of TNF-α may be responsible for dysfunction of hippocampus. As TNF-α increased in CSF and in claret following SNI, it is not surprising that the LTP was inhibited in bilateral hippocampus. The delayed and bilateral inhibition of hippocampal LTP induced past increase in TNF-α might contribute to memory deficits. However, nosotros too institute that genetic deletion of TNFR1 could not completely prevent the inhibitory effect of SNI on LTP, as in TNFR1 KO mice the potentiation induced past HFS lasted for <1 h in SNI group, which was shorter than that in sham grouping (for up to 5 h). Therefore, autonomously from TNF-α, other factors may be too involved in inhibition of hippocampal LTP post-obit peripheral nervus injury.
How could peripheral nerve injury increase TNF-α in the hippocampus? As peripheral nerve injury upregulates TNF-α inside 3 h in DRG (Rothman et al, 2009) and in the present study simply peripheral nerve (axons of DRG neurons and motor neurons) was injured, we speculated that TNF-α might increase in DRG at first. Previously, nosotros have shown that TNF-α promotes itself product via activation of nucleus factor-κB in DRG and in spinal dorsal horn (Wei et al, 2007). Information technology is possible that TNF-α released from DRG and spinal dorsal horn might diffuse into CSF and trigger the production of TNF-α in other brain regions, including hippocampus, by the aforementioned autocrine mechanism. Alternatively, peripheral nerve injury may increase the permeability of blood brain barrier, allowing hematogenous macrophages and CD4+ T cells to invade the fundamental nervous system (Cao et al, 2009; Cao and DeLeo, 2008; Zhang et al, 2007). Both the autocrine of TNF-α and the infiltration of immunocytes may contribute to the persistent increment in TNF-α in hippocampus. Further study is needed to elucidate this upshot.
Clinically, the retentiveness damage was intensively studied in the patients with fibromyalgia and low back pain (Hart et al, 2000). As fibromyalgia and neuropathic pain display similar clinical features (see Offenbaecher and Ackenheil, 2005 for a review) and share overlapping pathophysiological processes, it has been proposed that the two diseases may be variations of the same status (come across Maletic and Raison, 2009 for a review). The central mechanisms of low back pain are also similar to those of neuropathic pain (Audette et al, 2005). Importantly, it has been shown that serum concentration of TNF-α is increased in the patients with both fibromyalgia and low back hurting, and is reduced post-obit multidisciplinary treatment of pain (Wang et al, 2008a, 2008b). Therefore, this data obtained in animal model of chronic neuropathic pain may explicate the memory deficits in the patients with some forms of chronic hurting.
In summary, following peripheral nerve injury the increased TNF-α may not only contribute to the chronic hurting, but also to memory deficits past dysfunction of hippocampus. Therefore, the drugs targeting to TNF-α and its downstream molecules may treat both of the disorders.
References
-
Alloway TP, Gathercole SE, Kirkwood H, Elliott J (2009). The cerebral and behavioral characteristics of children with depression working memory. Child Dev 80: 606–621.
-
Audette JF, Emenike E, Meleger AL (2005). Neuropathic depression back pain. Curr Pain Headache Rep 9: 168–177.
-
Awh E, Vogel EK, Oh SH (2006). Interactions between attention and working memory. Neuroscience 139: 201–208.
-
Bruel-Jungerman Eastward, Davis South, Laroche South (2007). Encephalon plasticity mechanisms and retention: a political party of four. Neuroscientist 13: 492–505.
-
Butler MP, O'Connor JJ, Moynagh PN (2004). Dissection of tumor-necrosis gene-alpha inhibition of long-term potentiation (LTP) reveals a p38 mitogen-activated protein kinase-dependent mechanism which maps to early-merely not tardily-phase LTP. Neuroscience 124: 319–326.
-
Cao L, DeLeo JA (2008). CNS-infiltrating CD4+ T lymphocytes contribute to murine spinal nerve transection-induced neuropathic pain. Eur J Immunol 38: 448–458.
-
Cao L, Palmer CD, Malon JT, De Leo JA (2009). Disquisitional role of microglial CD40 in the maintenance of mechanical hypersensitivity in a murine model of neuropathic pain. Eur J Immunol 39: 3562–3569.
-
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994). Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53: 55–63.
-
Cunningham AJ, Murray CA, O'Neill LA, Lynch MA, O'Connor JJ (1996). Interleukin-ane beta (IL-1 beta) and tumour necrosis gene (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neurosci Lett 203: 17–20.
-
Decosterd I, Woolf CJ (2000). Spared nervus injury: an beast model of persistent peripheral neuropathic pain. Pain 87: 149–158.
-
Dick BD, Rashiq S (2007). Disruption of attending and working memory traces in individuals with chronic pain. Anesth Analg 104: 1223–1229.
-
Eccleston C (1995). Chronic pain and distraction: an experimental investigation into the function of sustained and shifting attention in the processing of chronic persistent hurting. Behav Res Therapy 33: 391–405.
-
Etherton JL, Bianchini KJ, Ciota MA, Heinly MT, Greve KW (2006). Pain, malingering and the WAIS-III working retentivity alphabetize. Spine J half dozen: 61–71.
-
George A, Buehl A, Sommer C (2004). Wallerian degeneration after beat injury of rat sciatic nerve increases endo- and epineurial tumor necrosis factor-alpha protein. Neurosci Lett 372: 215–219.
-
George A, Marziniak Yard, Schafers G, Toyka KV, Sommer C (2000). Thalidomide treatment in chronic constrictive neuropathy decreases endoneurial tumor necrosis factor-blastoff, increases interleukin-x and has long-term furnishings on spinal cord dorsal horn met-enkephalin. Hurting 88: 267–275.
-
Gould E, Leuner B (2010). Structural plasticity and hippocampal function. Annu Rev Psychol 67: 111–140.
-
Hart RP, Martelli MF, Zasler ND (2000). Chronic pain and neuropsychological operation. Neuropsychol Rev 10: 131–149.
-
Hu Y, Yang J, Hu Y, Wang Y, Li W (2010). Amitriptyline rather than lornoxicam ameliorates neuropathic pain-induced deficits in abilities of spatial learning and memory. Eur J Anaesthesiol 27: 162–168.
-
Ignatowski TA, Covey WC, Knight PR, Severin CM, Nickola TJ, Spengler RN (1999). Brain-derived TNFalpha mediates neuropathic pain. Brain Res 841: 70–77.
-
International Association for the Written report of Pain (1986). Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Pain three: 1–225.
-
Kesner RP (2007). Behavioral functions of the CA3 subregion of the hippocampus. Learn Mem 14: 771–781.
-
Legrain V, Damme SV, Eccleston C, Davis KD, Seminowicz DA, Crombez Thou (2009). A neurocognitive model of attention to hurting: behavioral and neuroimaging bear witness. Pain 144: 230–232.
-
Leite-Almeida H, Almeida-Torres Fifty, Mesquita AR, Pertovaara A, Sousa N, Cerqueira JJ et al (2009). The impact of age on emotional and cerebral behaviours triggered by experimental neuropathy in rats. Pain 144: 57–65.
-
Leuner B, Shors TJ (2004). New spines, new memories. Mol Neurobiol 29: 117–130.
-
Leung LW (1979). Orthodromic activation of hippocampal CA1 region of the rat. Encephalon Res 176: 49–63.
-
Maletic Five, Raison CL (2009). Neurobiology of low, fibromyalgia and neuropathic hurting. Front Biosci fourteen: 5291–5338.
-
Noguchi K, Obata K, Dai Y (2004). Changes in DRG neurons and spinal excitability in neuropathy. Novartis Found Symp 261: 103–110.
-
Offenbaecher K, Ackenheil M (2005). Electric current trends in neuropathic pain treatments with special reference to fibromyalgia. CNS Spectr ten: 285–297.
-
Pickering M, Cumiskey D, O'Connor JJ (2005). Actions of TNF-alpha on glutamatergic synaptic transmission in the central nervous system. Exp Physiol 90: 663–670.
-
Pickering M, O'Connor JJ (2007). Pro-inflammatory cytokines and their effects in the dentate gyrus. Prog Brain Res 163: 339–354.
-
Ribeiro RA, Vale ML, Ferreira SH, Cunha FQ (2000). Analgesic result of thalidomide on inflammatory hurting. Eur J Pharmacol 391: 97–103.
-
Rothman SM, Huang Z, Lee KE, Weisshaar CL, Winkelstein BA (2009). Cytokine mRNA expression in painful radiculopathy. J Pain 10: 90–99.
-
Schafers M, Brinkhoff J, Neukirchen South, Marziniak Yard, Sommer C (2001). Combined epineurial therapy with neutralizing antibodies to tumor necrosis factor-blastoff and interleukin-ane receptor has an condiment outcome in reducing neuropathic pain in mice. Neurosci Lett 310: 113–116.
-
Sekiguchi Thousand, Sekiguchi Y, Konno SI, Kobayashi H, Homma Y, Kikuchi SI (2009). Comparison of neuropathic pain and neuronal apoptosis following nervus root or spinal nervus compression. Eur Spine J xviii: 1978–1985.
-
Shamash S, Reichert F, Rotshenker S (2002). The cytokine network of Wallerian degeneration: tumor necrosis gene-blastoff, interleukin-1alpha, and interleukin-1beta. J Neurosci 22: 3052–3060.
-
Sommer C, Lindenlaub T, Teuteberg P, Schafers Yard, Hartung T, Toyka KV (2001). Anti-TNF-neutralizing antibodies reduce pain-related behavior in two different mouse models of painful mononeuropathy. Brain Res 913: 86–89.
-
Spengler RN, Sud R, Knight PR, Ignatowski TA (2007). Antinociception mediated past blastoff(2)-adrenergic activation involves increasing tumor necrosis factor alpha (TNFalpha) expression and restoring TNFalpha and alpha(two)-adrenergic inhibition of norepinephrine release. Neuropharmacology 52: 576–589.
-
Sunday HY, Lyons SA, Dobrunz LE (2005). Mechanisms of target-cell specific short-term plasticity at Schaffer collateral synapses onto interneurones vs pyramidal cells in juvenile rats. J Physiol 568: 815–840.
-
Tancredi 5, D'Arcangelo G, Grassi F, Tarroni P, Palmieri Thou, Santoni A et al (1992). Tumor necrosis gene alters synaptic transmission in rat hippocampal slices. Neurosci Lett 146: 176–178.
-
Tobinick E (2009). Perispinal etanercept for neuroinflammatory disorders. Drug Discov Today xiv: 168–177.
-
Toth C, Lander J, Wiebe South (2009). The prevalence and affect of chronic pain with neuropathic pain symptoms in the general population. Hurting Med 10: 918–929.
-
Vago DR, Kesner RP (2008). Disruption of the direct perforant path input to the CA1 subregion of the dorsal hippocampus interferes with spatial working memory and novelty detection. Behav Brain Res 189: 273–283.
-
Wang H, Moser Yard, Schiltenwolf Chiliad, Buchner M (2008a). Circulating cytokine levels compared to pain in patients with fibromyalgia—a prospective longitudinal study over half-dozen months. J Rheumatol 35: 1366–1370.
-
Wang H, Schiltenwolf M, Buchner M (2008b). The function of TNF-alpha in patients with chronic low back pain-a prospective comparative longitudinal study. Clin J Pain 24: 273–278.
-
Wang Q, Wu J, Rowan MJ, Anwyl R (2005). Beta-amyloid inhibition of long-term potentiation is mediated via tumor necrosis factor. Eur J Neurosci 22: 2827–2832.
-
Wei XH, Zang Y, Wu CY, Xu JT, Xin WJ, Liu XG (2007). Peri-sciatic assistants of recombinant rat TNF-alpha induces mechanical allodynia via upregulation of TNF-alpha in dorsal root ganglia and in spinal dorsal horn: the role of NF-kappa B pathway. Exp Neurol 205: 471–484.
-
Xu JT, Xin WJ, Zang Y, Wu CY, Liu XG (2006). The role of tumor necrosis factor-alpha in the neuropathic pain induced by lumbar v ventral root transection in rat. Hurting 123: 306–321.
-
Xu L, Anwyl R, Rowan MJ (1997). Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature 387: 497–500.
-
Yamanaka H, Obata M, Fukuoka T, Dai Y, Kobayashi K, Tokunaga A et al (2004). Tissue plasminogen activator in primary afferents induces dorsal horn excitability and pain response after peripheral nervus injury. Eur J Neurosci 19: 93–102.
-
Zhang J, Shi XQ, Echeverry S, Mogil JS, De Koninck Y, Rivest S (2007). Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci 27: 12396–12406.
-
Zou LB, Yamada Chiliad, Tanaka T, Kameyama T, Nabeshima T (1998). Nitric oxide synthase inhibitors impair reference memory formation in a radial arm maze chore in rats. Neuropharmacology 37: 323–330.
Acknowledgements
Nosotros thank William D Willis Jr for editing the English of the manuscript. This work was supported by grants from the National Natural Science Foundation of China (no: 30770705) to XGL and (30630026) to GL, the Ministry building of education of People's republic of china (20060558001) to XGL, and National Bones Inquiry Plan of People's republic of china (2009CB941303) to GL.
Author information
Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional data
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
PowerPoint slides
Rights and permissions
Most this article
Cite this article
Ren, WJ., Liu, Y., Zhou, LJ. et al. Peripheral Nerve Injury Leads to Working Memory Deficits and Dysfunction of the Hippocampus by Upregulation of TNF-α in Rodents. Neuropsychopharmacol 36, 979–992 (2011). https://doi.org/10.1038/npp.2010.236
-
Received:
-
Revised:
-
Accepted:
-
Published:
-
Upshot Appointment:
-
DOI : https://doi.org/10.1038/npp.2010.236
Keywords
- spared nerve injury
- chronic neuropathic pain
- working memory
- long-term potentiation
- presynaptic boutons
- tumor necrosis gene-α
Further reading
Source: https://www.nature.com/articles/npp2010236
0 Response to "1 Tnf-ãžâ± and Neuropathic Pain - a Review"
Post a Comment